External or condom catheters are designed for men with incontinence in which the sheath rolls over the penis like a condom. Can be secured with tape or adhesive and are easy to use.
Intermittent catheters are for those who do not need continuous catheterization but must empty out bladder several times a day. There are straight tip, PVC, hydrophilic, coude t...
Incontinence is the involuntary loss of bladder and bowel control. Incontinence can range in severity from a small leak to complete loss of bladder or bowel control.
Penile Clamps are a safe non-invasive procedure that requires no surgery or drugs to treat Male Incontinence.Choose from Bard Zipser Penile Clamp, Bard Hyams Penile Clamp, Bard...
Pessaries are an effective tool for managing pelvic organ prolapse without surgery and are available in different sizes and with/without support. We offer Shaatz Pessary,Donut P...
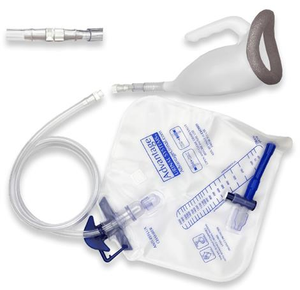
Male urinal system usually consists of a urinal and a large capacity reservoir bag connected through a tubing. Urinal is used to drain urine to the drainage bag via tubing.
Fertility testing is the process by which fertility is assessed, both generally and also to find the "fertile window" in the menstrual cycle. The onset of puberty is t...
Urine Leg Bags are useful for the temporary storage of urine in case of urinary incontinence (leakage), urinary retention or surgery. They are mostly drainable.